All-optical Electrophysiology
Problems
One of the current problems regarding electrophysiology is related to the use of electrodes, which limits both the specificity of cells to excite or from which to read a signal. Another limitation is related to the impossibility of inhibiting neural activity with electrodes, which must be done pharmacologically. Finally, another problem concerns resolution: in fact, it is not possible to distinguish the membrane potential of a neuron from that of its neighbours with the use of electrodes.
Technology
The use of membrane channels inserted in the proteins of neurons through genetic modifications, whose spatial conformation depends on the interaction with light, has recently opened a field called Optogenetics by which neurons can both be excited and inhibited through photons. Furthermore, the genetic transfection technique allows the selection of neurons to excite or inhibit. By coupling this labeling technique with a second based on Calcium Ion concentration signal reporters (indirect reporters of electrical activity), it is also possible to measure the electrical activity induced through the photoactivable channels.
Outcomes and impact
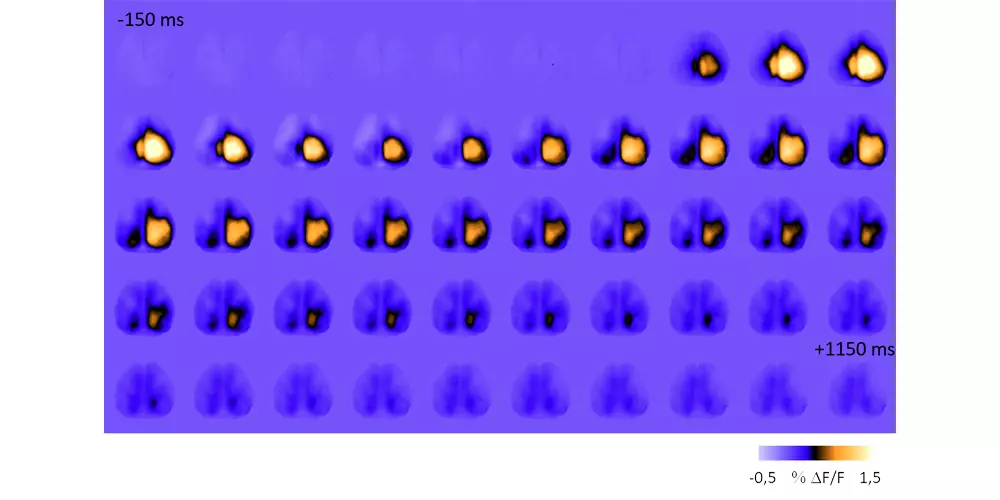
The possibility of simultaneously activating/inhibiting and recording the electrical activity of the neural circuit, using only photons and not electrodes, is called All-Optical Electrophysiology and has the advantage of being neuron-specific, with a much higher resolution than classic electrophysiology and allowing to access many more brain areas.
More details
The use of light to stimulate and readout neuronal activity has several advantages, such as the noninvasiveness and the possibility to target with high spatial and temporal precision specific groups of neurons. In the past decade, there was a parallel technological revolution for both functional indicators and optogenetic actuators. The development of genetically encoded activity sensors brought us close to single-action-potential sensitivity and, on the other side, optogenetics allows us to stimulate or inactivate defined populations of neurons with single-cell precision on the millisecond time-scale. However, achieving an all-optical interrogation of neural circuits by combining these tools is still arduous, mainly due to the spectral overlap between actuators and indicators. To this aim we are developing different all-optical approaches:
– In mouse models, we combine red-shifted calcium indicators with blue-activated opsins for simultaneous imaging and stimulation of large cortical functional areas. Moreover, we are developing new devices (optoelectrodes) to combine simultaneously cortical imaging recording, subcortical optogenetic stimulation and electrophysiological recordings.
– In zebrafish models, we adopt an orthogonal approach (blue-excited calcium indicator and red-shifted opsin) to perform all-optical electrophysiology. Indeed, owing to the zebrafish larvae optical transparency, we are able to perform simultaneous high-speed whole-brain calcium imaging and three-dimensional individual neuron optogenetic stimulation, using a custom-made light-sheet microscope and a novel custom light-steering module, respectively. The use of two-photon excitation, both for imaging and optogenetic stimulation, allows us to minimize spectral crosstalk and to finely tailor the excitation pattern.