Large Volumetric Mapping with light-sheet microscopy
Problems
The problem concerns the structural mapping of large portions of the brain up to the entire brain, both for animals and human. The aim is to correlate structures at the level of a few hundred nanometers with structures at larger scales. This correlation is fundamental since it behaves at an integrated level both structurally and functionally.
Technology
The main problem to be solved is to be able to see both the single detail and the entire structure in the same 3D image. To do so, the optical dynamic range of the system’s imaging capability must be pushed to extremes.
Outcomes and impact
The impact of this technology is related to the entire mapping of the brain, specifically highlighting the various cellular and axonal structures that compose it, creating for the first time a 3D image of the brain with a resolution of 10,000 or 100,000 higher than currently available techniques such as MRI.
More details
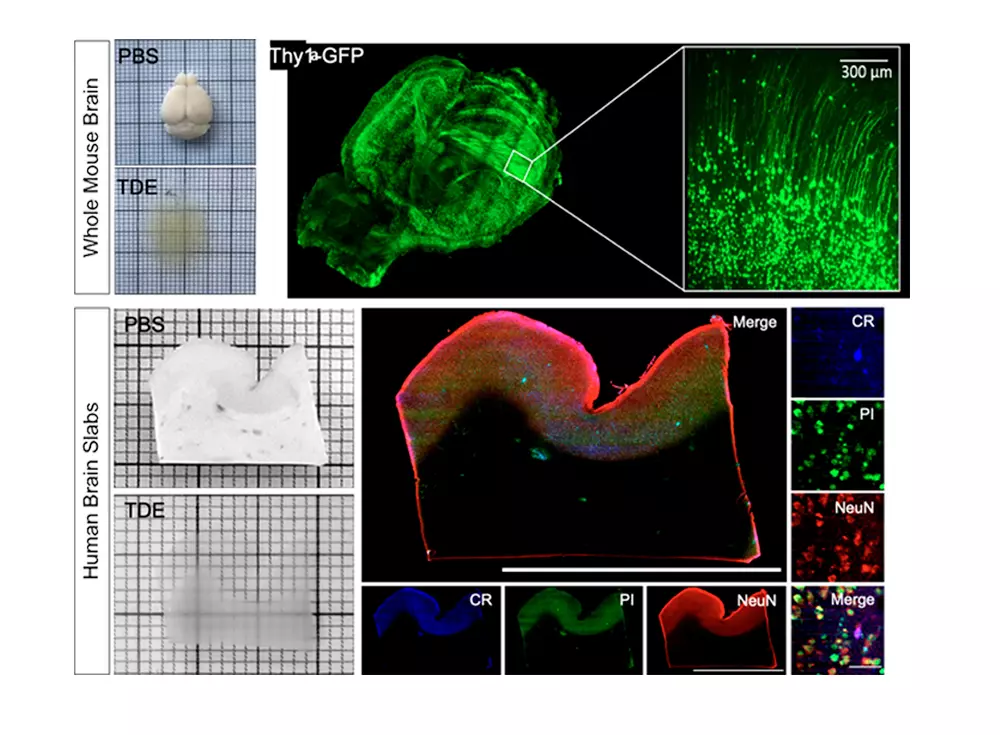
We leverage the intrinsic optical sectioning, high contrast and direct fast 2D image recording of confocal light-sheet fluorescence microscopy (CLSFM) to obtain with cellular resolution three-dimensional reconstructions of large intact neuronal networks for an improved understanding of the mice and human brain structure. Both whole fixed mice brains and centimeter-sized human brain samples pose substantial biological and technical challenges due to their extension and inhomogeneous optical properties, consequently an optimized preparation protocol and dedicated CLSFM setup has been developed for each.
Mice
We are optimizing CLSM for imaging cm-sized samples with micrometer resolution and high image contrast. We recently devised RAPID, a method for real-time correction of the defocus introduced by the specimen itself. Further, we are investigating methods – including non-Gaussian beams and/or advanced pivoting strategies – to mitigate the shadowing artefacts typical of light-sheet illumination. We exploit the high resolution of our system to map cytoarchitecture in the whole mouse brain, and to reconstruct the vascular structure at the capillary level.
Human
We are developing a refined optical clearing method (SWITCH-TDE) to obtain transparent post-mortem human brain samples that are macromolecule-permeable and compatible with fluorescence immunostaining, given the impossibility to use genetically encoded fluorescent probes. Immunocytochemical staining methods for marker of different interneuron types in the human neocortex are being optimized in order to obtain morphology and molecular type data.
Imaging is performed by a custom dual-view inverted confocal light sheet fluorescence microscope (di2CLSFM). Our instrument, by leveraging the two orthogonal views, provides an almost isotropic micrometric resolution along the three optical axes and, due to its high volumetric speed of 8 mm3/minute, allows to capture centimeter-sized samples in up to four fluorescence bands within one hour. Finally, the acquired multi-TeraByte datasets demand advanced automatic image analysis pipelines for robust segmentation in order to extract multilevel maps of quantitative cell distributions and morphologies, categorized by type.